15+ Why Is Ch3F Polar
Check out a sample QA here See Solution. Web Hi everyone here we have a question asking us to describe why chloroform is a polar compound.
Is Ch3f Polar Or Non Polar Quora
Polarity refers to having an overall charge positive or negative in a molecule.

. For this reason this. Despite having a tetrahedron geometry which usually gives a non polar. The electronegativity difference value of carbon and fluorine atom is higher than 05 this.
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Web Solution for Explain why CH3F is a polar molecule while CF4 is non-polar. Web Explain why the molecule CH3F is polar.
The C-H bond of the CH3F molecule becomes nonpolar in nature. Answer Request Follow 2 1 Answer Sort Recommended Rajarshi Panigrahi NET GATE qualified in Chemistry 2 y Dipole moment. Web This problem has been solved.
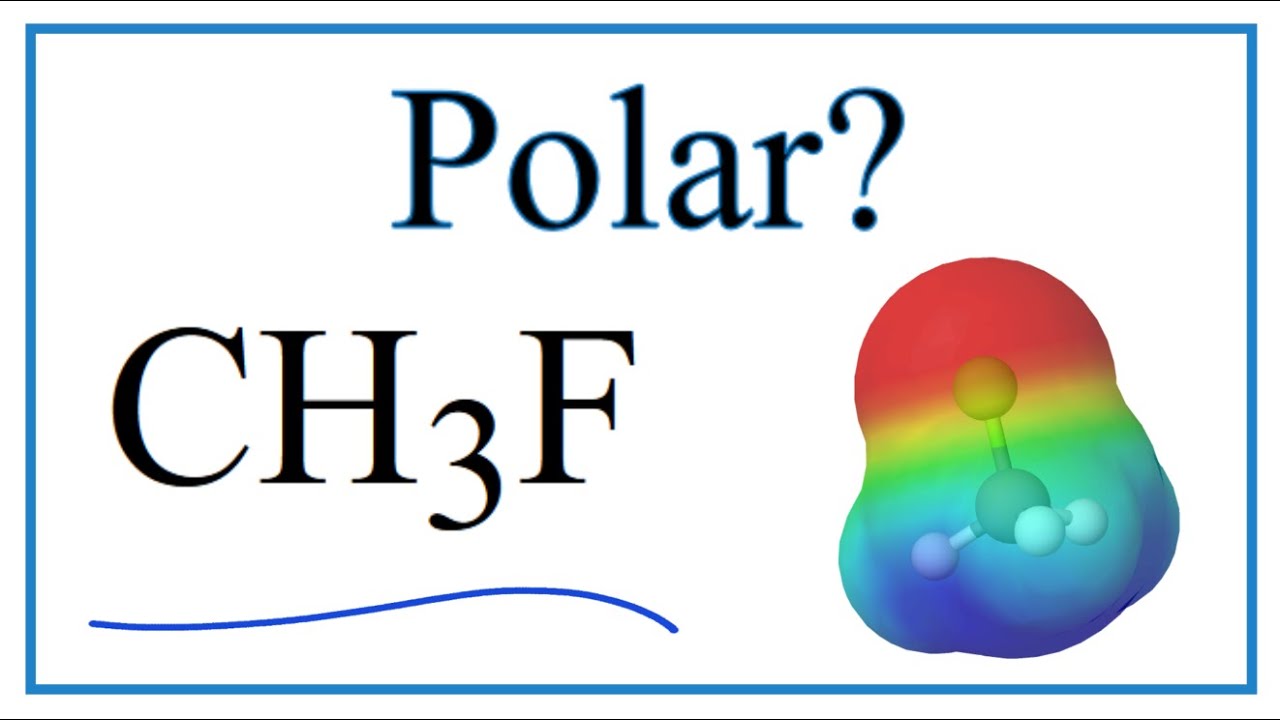
Is CH3F polar or non-polar and its Dipole moment CH3F has non zero dipole moment because the Fluorine atom has more electronegativity than carbon. Web CH3F is a POLAR molecule because the C-F bond present in the molecule is polar which causes the partial positive ẟ and partial negative ẟ- charge to appear on. Web Transcribed Image Text.
Web CH3F is polar. Expert Solution Want to see the full answer. Web However to determine if CH3F is polar we must consider the molecular geometry.
Wiki User 2011-01-17 230956 This. Web CH3F is a polar molecule even though the tetrahedral geometry often leads to nonpolar molecules. A polar molecule results from an unequalunsymmetrical sharing of valence.
It is an alkyl halide which has four sigma b. Web Why CH3Cl is more polar than CH3F. Explain why CH3F is a polar molecule while CF4 is non-polar.
Web The polarity of the CH3F molecule is due to the difference in electronegativity of the atoms. CH3F is an organic compound which contains four single bonds. See Answer Explain why the molecule CH3F is polar.
Web The molecules is perfectly symmetrical so every electron pair on each fluorine cancels out the electron pairs of every other fluorine. Web The C-F bond of the CH3F molecule becomes polar in nature due to this difference in electronegativity value. View the full answer Transcribed image text.
Using electronegativity one can. There are differences in electronegativity which are not canceled by the molecules tetrahedral shape.
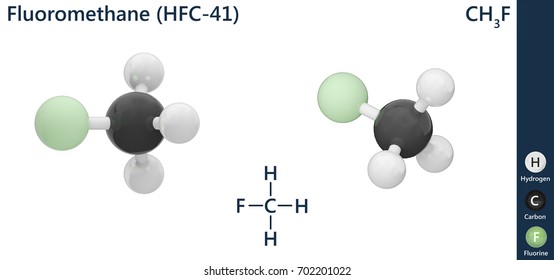
6 Ch3f Images Stock Photos Vectors Shutterstock
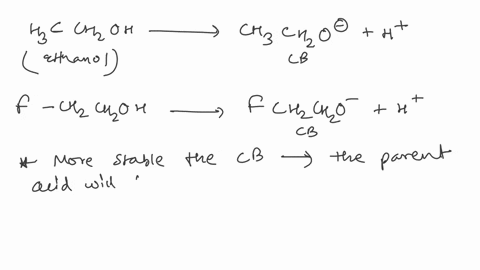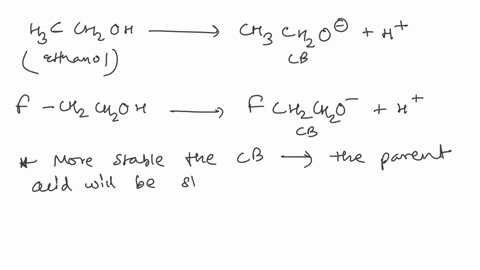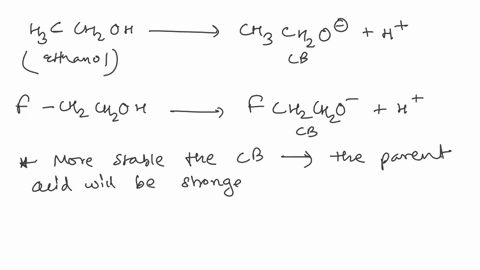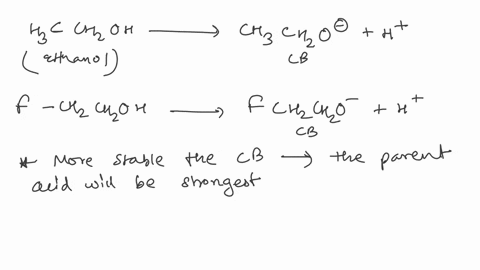
Solved Ch3f Is A Polar Molecule Even Though The Tetrahedral Geometry Often Leads To Nonpolar Molecules Explain
Why Is Ch3f Polar In Nature Quora

Is Ch3f Fluoromethane Polar Or Non Polar Youtube

Is Ch3f Polar Or Nonpolar Techiescientist

Why The Dipole Moment Of Ch3f Is Less Than Ch3cl Although F Is More Electronegative Than Cl By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
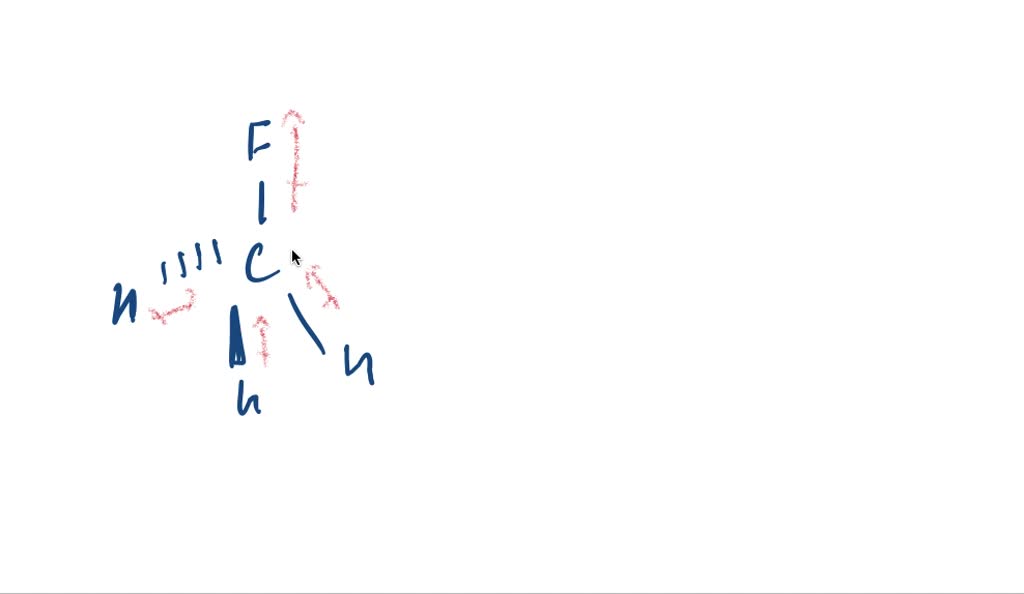
Solved Ch3f Is A Polar Molecule Even Though The Tetrahedral Geometry Often Leads To Nonpolar Molecules Explain
Best Overview Is Ch3f Polar Or Nonpolar Science Education And Tutorials

Explain Why Ch3f Is A Polar Molecule Even Though The Tetrahedral Geometry Often Leads To Nonpolar M Youtube

Is Ch3f Polar Or Nonpolar Polarity Of Methyl Fluoride

Best Overview Is Ch3f Polar Or Nonpolar Science Education And Tutorials

Bonding And Vsepr Theory Structures Of Solids And Liquids Intermolecular Attractions Ppt Download

Is Ch3f Polar Or Nonpolar Techiescientist
Is Ch3f Fluoromethane Polar Or Non Polar Youtube
Solved Ch3f Is A Polar Molecule Even Though The Tetrahedral Geometry Often Leads To Nonpolar Molecules Explain

Solved Ch3f Is A Polar Molecule Even Though The Tetrahedral Geometry Often Leads To Nonpolar Molecules Explain
The Dipole Moment Of Ch3f Is 1 85d And That Of Cd3f Is 1 86d Why Quora